Ordering Options
NGS SARS-CoV-2 TestingUPIP (Urine Pathogen ID Panel)
RPIP (Respiratory Pathogen ID Panel)
Turnaround Time*
NGS: ≤ 24 hours UPIP: 48 hours RPIP: 72 hoursCost
Please call for detailsResources
* Turnaround time starts when sample is received at the laboratory.
Covid-19Sequencing
Respiratory
Toxicology
Urinary Tract
Carrier Genetics
Gastrointestinal
MicroBiology
Sexually Transmitted Infection
Toe Nail
Wound Infection
Chemistry
What is NGS?
Next-generation sequencing (NGS) has revolutionized genomic research in the last two decades. NGS offers ultra-high throughput, scalability, and speed by sequencing millions of small DNA fragments in parallel. This technology has empowered scientists to decode the entire genomic sequence of a given DNA sample, be it uniform genomic content, such as the human genome, or non-homogeneous DNA derived from multifarious cell populations, such as microbial communities, known as metagenomics analysis.
What is Precision Metagenomics?
NGS has revolutionized the field of oncology and inherited diseases and ushered in the era of precision medicine (treatment customized for a group of patients based on their genetic information). Likewise, precision metagenomics is the application of NGS to customize the management of infectious diseases based on the genetic makeup of the microbiome.
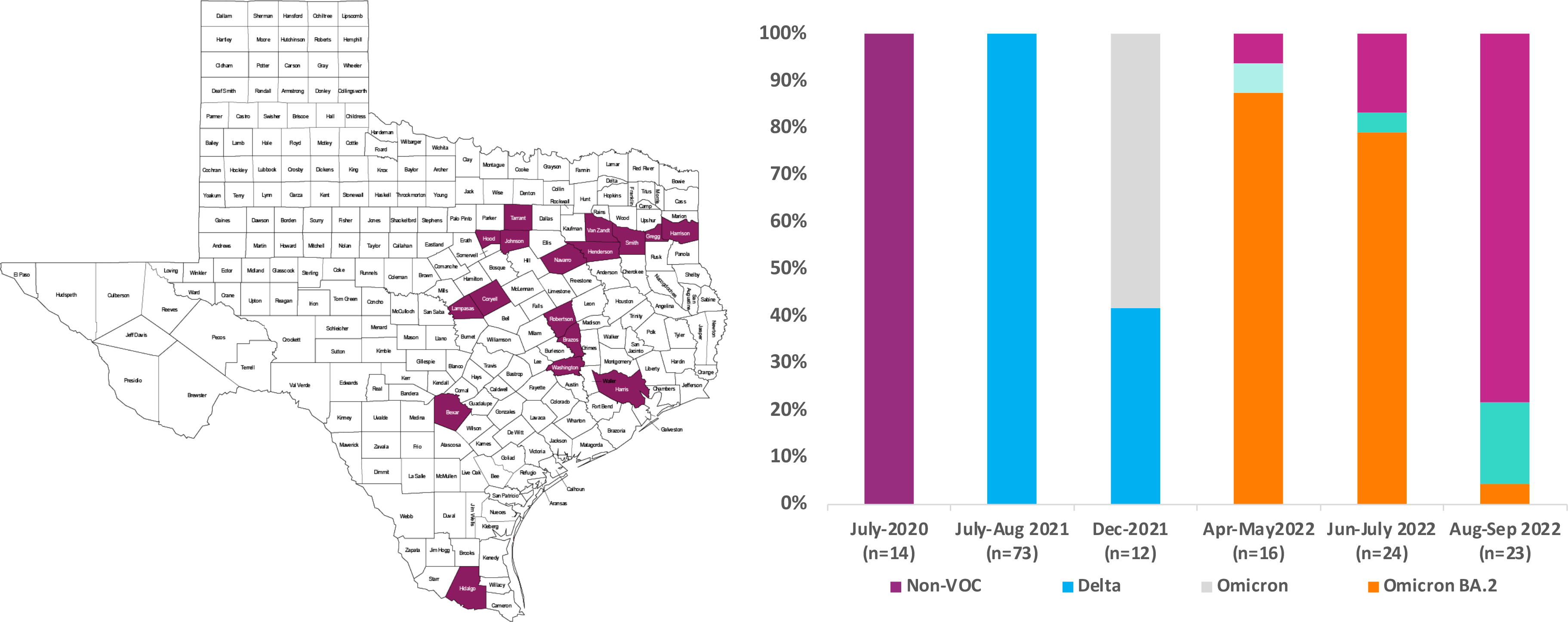
Advanta Genetics has adopted NGS for deciphering the microbial communities of respiratory and urinary infections and detecting the COVID variants in the east Texas region.
Rapid classification and tracking of emerging variants of the severe acute respiratory syndrome (SARS) SARS-CoV-2 are critical for understanding its transmission dynamics and developing strategies for severing the transmission chain. Clinical manifestation of the infection is influenced by comorbidities such as age, immune status, diabetes, and the infecting variant. Thus, clinical management may differ for new variants. But currently, there is no FDA-approved laboratory test for detecting SARS-CoV-2 variants.
At Advanta Genetics, we have fully validated the Illumina COVIDSeq™ assay as a laboratory-developed test in line with CLIA and CAP guidelines. Advanta is tracking the evolutions of SARS-Cov-2 variants in East Texas, and Advanta COVIDSeq is also validated for reporting the COVID variants for potential clinical application.
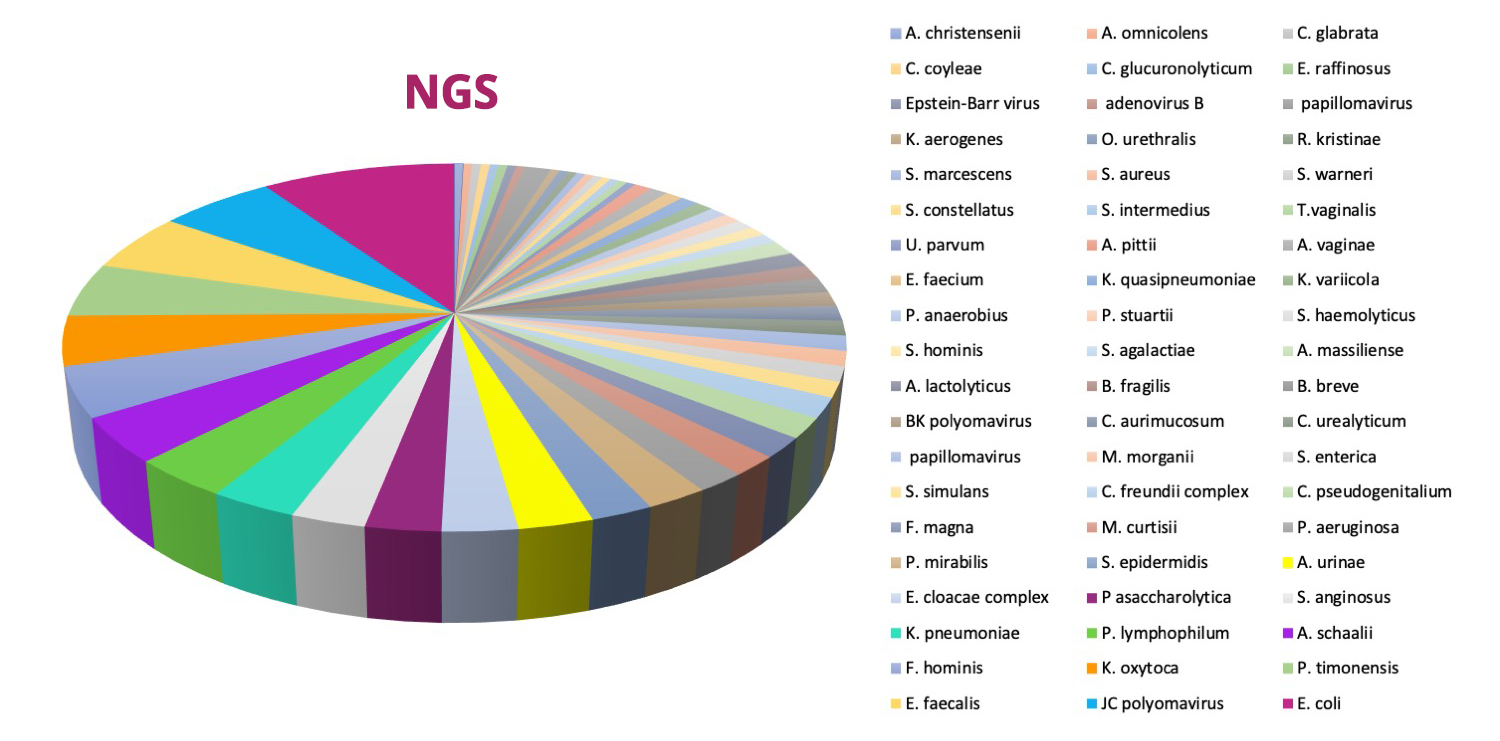
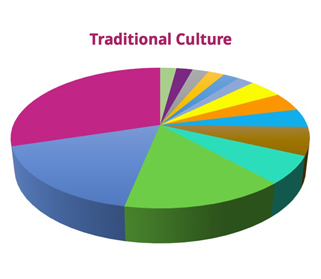
Timely and accurate identification of uropathogens is critical to manage urinary tract infections and limit antimicrobial resistance development and spread. However, the traditional culture method is inadequate for clinical diagnosis, and about 20% of women with UTI symptoms have a negative urine culture.
- NGS can provide a holistic picture of the microbial community in the urine samples, including bacterial, viral, and fungal pathogens.
- Accurate diagnosis = effective treatment and patient outcomes
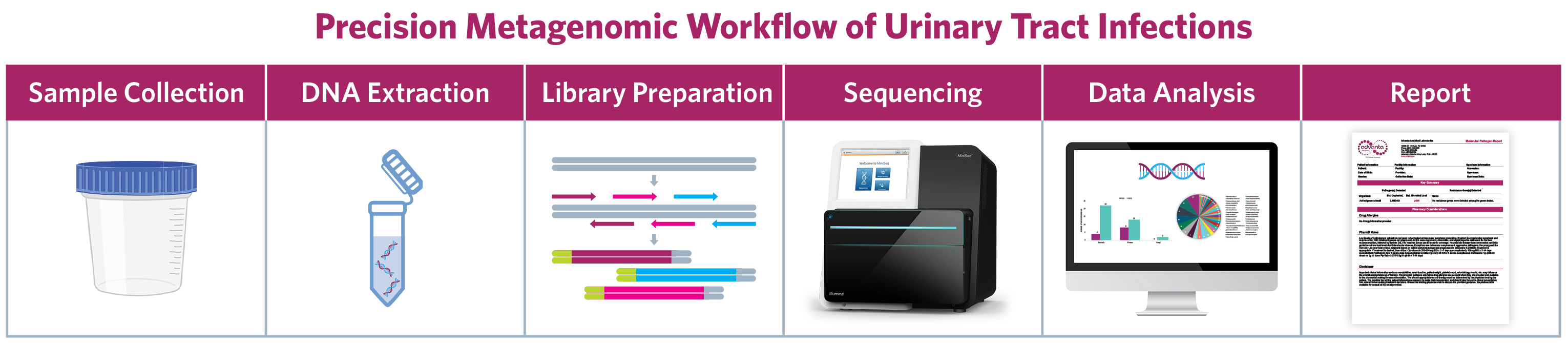
- Advanta UPIP mNGS test detects and quantifies 135 bacteria, 35 viruses, 14 fungi, and 7 parasites based on target-enriched next-generation sequencing (NGS) of microbial genome sequences.
- Accurate identification of clinically relevant uropathogens in 2 days
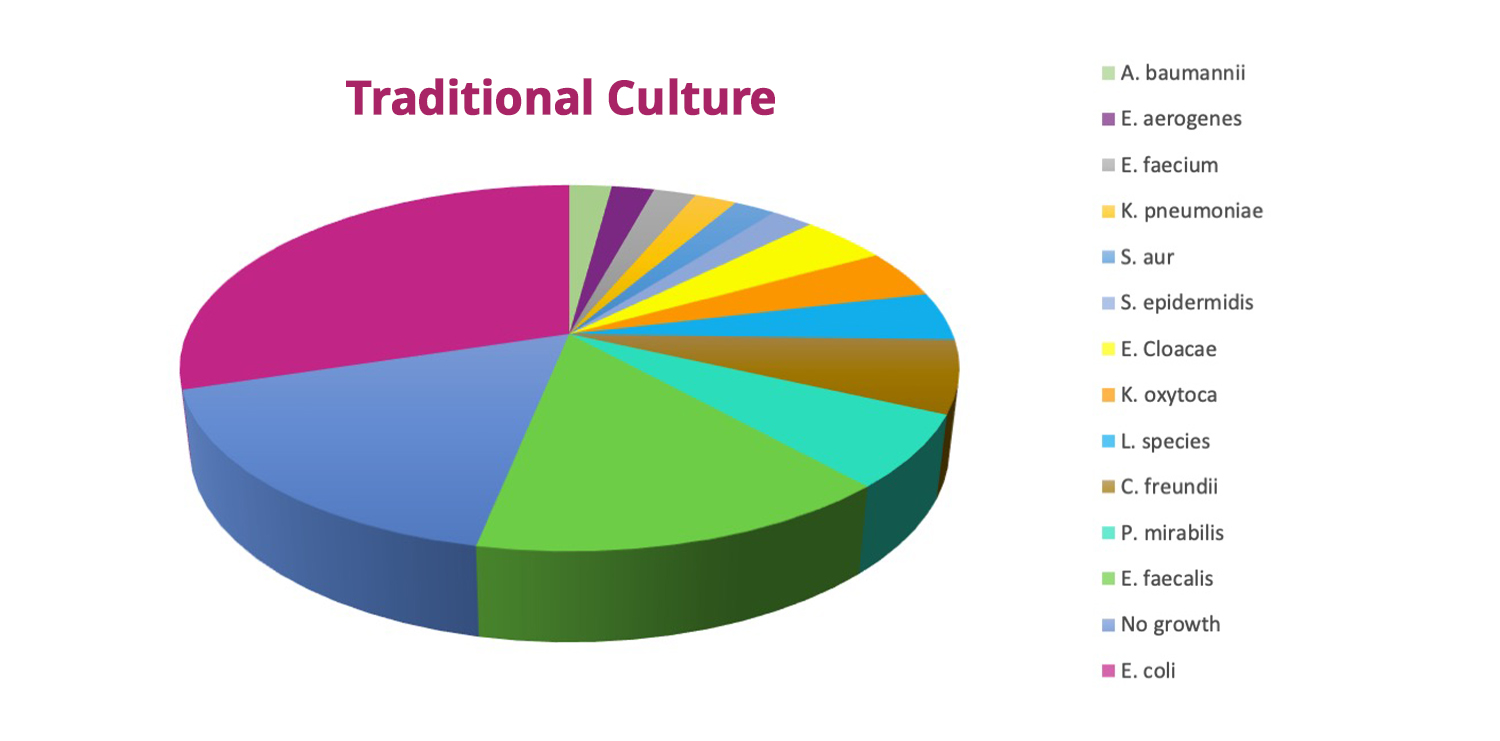
- Among 47 urine samples processed, Advanta mNGS identified about 60 uropathogens compared to 13 by standard urine culture.
- Respiratory infections are still a leading cause of mortality and morbidity.
- The limited ability of traditional pathogen detection methods results in misdiagnosis or inappropriate treatment.
- Rapid and accurate pathogen identification enables targeted treatment, minimizes broad-spectrum antibiotic overuse, and accelerates patient recovery.
- Precision medicine requires precision diagnosis.
- Target-independent metagenomic next-generation sequencing (mNGS) analysis is a powerful tool for precision diagnosis, omitting the guesswork that leads to much of the under or overtreatment of infectious disease.
- Metagenomic next-generation sequencing (mNGS) technology has the potential to accurately identify respiratory (co)infections without a priori knowledge aimed at broadening pathogen discovery, shortening detection time for certain microorganisms, and with detection strength less affected by past antibiotic exposure.
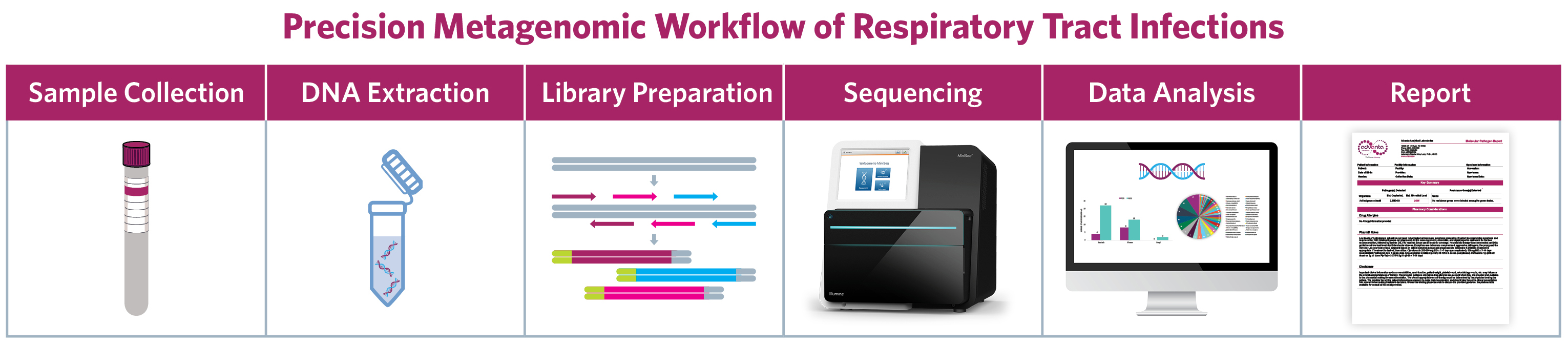
- Advanta RPIP mNGS test detects and quantifies 187 bacteria, 42 viruses, and 53 fungi based on target-enriched next-generation sequencing (NGS) of microbial genome sequences.
- Accurate identification of clinically relevant respiratory pathogens in 3 days.
We Love Genetic Research!
Currently, Advanta scientists are engaged in multiple collaborative research projects with educational and healthcare entities across the United States. We are particularly interested in the novel use of NGS for respiratory infections, joint infections, and urinary tract infections. Please reach out to us if you have interest in collaborating with Advanta on genetic research.
Advanta Research Laboratory research@aalabs.com